I described the key factors to be considered when selecting a model species for neuroscience research, and using examples from the literature, discuss how drosophila, zebrafish and mice meet these criteria
Body
describe the key factors to be considered when selecting a model species for neuroscience research, and using examples from the literature, discuss how drosophila, zebrafish and mice meet these criteria
Throughout the history of scientific research, animals have been used countless times. The earliest written record is that Aristotle (384 to 322 BC) and Erasistratus (304 to 258 BC), two early Greek physician-scientists conducted experiments on animals (Cohen and Loew 1984). In recent years, there is some new archaeological evidence to suggest that humans tried to operate a Neolithic surgery performing trepanation on a cow in 3400-3000 BCE (Ramirez and Froment, 2018). No matter what the behind meaning is, this action may be regarded as the first attempt at animal experiments in human history. When it comes to the modern era, as model biology rides on the locomotive of the rapid development of modern science, model animals become an essential topic in biology. August Krogh (1929), the 1920 Nobel Laureate in Physiology and Medicine, famously and succinctly articulated the selection of a model of a naturally occurring species, often known as the comparative approach: “For a large number of problems there will be some animal of choice or a few such animals on which it can be (most) conveniently studied”. Nowadays, the textbook definition of an animal model “as a living organism with an inherited, naturally acquired, or induced disease state that in one or more ways closely mimics the same phenomenon existing in man” has affected how researchers approach animal models (Wessler, 1976).
For neuroscience, several factors are supposed to be considered during selecting a model species. The criteria are stated by Davidson et al. (1987) as followed 9 points: 1) suitability as an analogue, 2) information transferability, 3) genetic uniformity, 4) biological background, 5) expense and accessibility, 6) generalizability for an outcome, 7) usability and adaptability of experimental manipulation, 8) environmental status, 9) moral involvement. Considering these criteria, model selection is mostly a matter of personal preference for individual scientists, who must then persuade the rest of the research world that their choice is sound. Many model animals, such as drosophila, zebrafish, and mice, share physiological, behavioural, and other features with humans, according to modern science. However, these rules have not treated model animals in many details. As far as Wright was concerned in 2002, the goal of an animal model specifies the requirements that it must meet in order to be considered legitimate. As a result, to decide the weights allocated to the different evaluation criteria, any model evaluation approach must consider the goals and needs that a model is designed to meet, as well as the questions it is anticipated to answer. Meanwhile, some argue that specific research should be conducted on intact, live organisms with as little experimental manipulation as feasible to avoid experimenter-induced artefact (Dow, 2007). Otherwise, the experiment, like Schrödinger’s cat (Schrödinger, 1935), will undermine the validity of the outcome. In 2009, a more systemic approach (fig 1) was put forward by van der Staay et. al. At the beginning, an ethical question must be asked: whether it is acceptable? The model evaluation process continues with the question of whether the data generated by the model is reliable and repeatable, implying that deficiencies must be reproducible and behavioural dysfunctions must be quantified using reliable procedures. The model’s face validity, construct validity is then discussed. After that, if the proposed model has predictive validity becomes another problem to be concerned. All these above validities reach requirements, external validity and generalizability of the model is the final step in the model evaluation cycle. Those standards are important, but as we mentioned before, each independent experiment requires various models. Therefore, we are going to discuss 3 general model animals, mice, zebrafish and drosophila as follows.
In the past decades, a lot of different types of model animals meeting these criteria were proposed and applied, some of which decay while others show strong vitality. PubMed search results by publication on the date (Fig 2) shows that the mice continue to be the powerhouse for bioscience. The other two model animals have also been used thousands of times. The very first reason why they can be the most common model animals is that they are all affordable, usability and adaptability. There is no doubt that the most significant shift in the last decades has been the dramatic increase in the use of mice in research (McCammon, 2015). Eckardt et al. (2011) argued that the mouse appears to be the most prevalent genetically altered animal model for studying novel therapeutic compounds for various illnesses at the moment, for the following reasons. As for genome conservation, 99 per cent of human genes have homologues in mice (Mouse Genome Sequencing Consortium et al., 2002). Therefore, generalizability for the outcome is doubtless accessible. The use of mouse models to evaluate pharmacological targets and develop effective and safe dose schemes for combination therapies in humans has proven successful. These scenarios all have one thing in common: they don’t seek out to fully simulate a disease or disease mechanisms, but rather to gather relevant functional data. In this case, rough modelling refers to an efficient method. As for new models involving the mouse, pathological processes in human-mouse chimaeras can be uncovered by combining cellular and whole-animal techniques. To create human and mouse hybrids, human iPSCs are injected into blastula of a mouse, or tissue from human is implanted into immune-deficient mice that are older (Eckardt et al., 2011). Due to evolutionary similarities and historical reasons, mice are estimated to be the most used species to model human diseases and many other diseases. However, it is not possible to fully replicate all symptoms for one certain strain. The details are also very important when doing experiments, which is why there are specific protocols.
The mouse is a good model animal with many advantages; however, it is not the only solution or the best choice for some questions. Both the basic macro-organization of the brain and cellular morphology are strikingly comparable in zebrafish and mammalian models, the mouse for example (Kalueff, Stewart & Gerlai, 2014). The intermediate complexity of Danio rerio makes it appropriate for brain study and drug testing. It has a genetic structure that is similar to primates and a physiology that is comparable to mammals. A genetically tractable species with a sequenced genome and a broad toolset for genetic alteration, the zebrafish, like a drosophila. (Elena, 2018). The genomes of zebrafish and mammals are highly similar, with the zebrafish genome containing more than 80% of human disease genes (Howe et al., 2013). McCammon (2015) holds the view that the zebrafish is a human-like animal model that blends experimental tractability with conservation. They are quite inexpensive to keep and maintain, and they generate a high number of embryos. A considerable standard of molecular, cellular, morphological, and developmental conservation, fast temporary hereditary tests, ability for editing gene, imaging of living organism, behaviours with characteristics, multiple-diseases research, and suitability for identifying putative medicines of chemical screening are among the zebrafish’s significant attributes for addressing psychiatric disorders. The zebrafish is an excellent model organism for studying the neurological underpinnings of natural behaviour. This has been made possible by a number of technologies, including new advances in animal monitoring, computational analysis of behaviour, functional imaging of the whole brain, and methods for specific circuits and genetic manipulation. (Orger and De Polavieja 2017). In conclusion, zebrafish genetics is best adapted to large amount transient analyses, especially variant analysis and genetic interactions. However, there are also substantial disadvantages to using zebrafish models in the neuroscience research. Even though combining chemical substances with water is a simple way to do pharmacological manipulations, Chemicals can be quickly metabolized through skin and gills, based on the surface area of a particular fish and gill activity, therefore such experiments couldn’t appropriately manage the medication amount taken (Rubinstein, 2006). Furthermore, from the results from Chatterjee and Gerlai (2009), zebrafish pharmacokinetic studies are still limited, and the amount of drug that reaches different target tissues is poorly explored, despite the fact that its presence and amount in the CNS can be confirmed using various chemo-analytical methods such as mass spectroscopy or high-performance liquid chromatography. The above reasons are partly why we introduce an insect as follows.
There is a smaller and simpler model animal that can be competent for a job the above two animals can’t replace. Drosophila melanogaster, a well-known model animal, was famous for the finding of the concept of heritable qualities being carried on chromosomes, as well as many other ground-breaking genetic findings (Morgan 1911). The genome of drosophila was sequenced as the first main for the first time in the modern era (Adams et al., 2000). But why is drosophila a suitable model for neuroscience? Elena et al. (2018) suggest that many of these species are excellent models for understanding the cellular circuits underlying behaviour and physiology, neurotransmission, sensory perception, and plasticity, as well as the cellular basis of learning and memory at the level of the individual cell, due to the simpler organisation of the invertebrate nervous system and the presence of many accessible neurons of surprisingly large sizes at landmark locations throughout the central nervous system. Another benefit of using drosophila as a model is that the life cycle of the fly is quite short. In 10 to 12 days at 25°C, a single viable mating pair can generate hundreds of genetically identical offspring. This is in contrast to standard mouse models, which generate just a few offspring every three to four months (Pandey, 2011). At the gene level, the fly has a number of distinguishing characteristics that make it an appealing model to examine. The genome of drosophila is entirely sequenced and annotated, which encodes for more than 14,000 genes on totally four chromosomes, with three of four carrying the majority of the genome. Almost 75 per cent of genes linked to disease in humans have functioning orthologs in the drosophila, according to estimates (Reiter et al., 2001). Of course, when transferring proteins like A-beta or alpha-synuclein to fruit flies to build a disease model, someone will question: if you transfer a protein that this organism does not have originally at all, there may be some symptoms. But what is the point and whether it is meaningful or not? Moreover, the evolutionary difference between this kind of creature and human beings is too far regarding the rodent. Based on this statement, it is generally believed that the drosophila is suitable for large-scale screening, for instance, RNAi or EMS mutagenesis belonging to reverse or forward screen and the existing resources are abundant. (Pandey 2011) Experiments such as imaging behaviour are easy to operate and easy to raise. There is a big gap, but at least the targets that are worthy of in-depth selection can be screened out in the early stage. However, with the emergence of new technologies such as RNAi in mice (Slobodan 2013), these advantages may gradually weaken, but it is undeniable that some people still make models of such small animals.
All in all, these model animals do a firm favourite to the development of neuroscience. On the other hand, the contributions to this issue show that a range of less common and, at times, more specialised animal model systems are used to make numerous breakthroughs in neuroscience. Our understanding of evolutionarily conserved core processes and adaptive solutions that are basic to CNS function across phyla has improved thanks to research employing this diverse set of models (Elena 2018). Advances in stimulus delivery, behavioural monitoring, and the measurement and modulation of brain activity have made model animals behaviour more accurate and experimentally accessible in naturalistic settings. The rapid pace of research in this area should result in more quantitative modelling approaches, a better understanding of behaviour ontogeny, and better optical tools, making mice, zebrafish and drosophila an appealing system not only for genetics and development research but also for investigating the neural circuit basis of complex behaviours (Orger and De Polavieja 2017). In neuroscience research, model animals will add a rich and colourful stroke to the annals of history. The next century will be illuminated as more and more new animal models are being designed and put into use. The building of biology is waiting for us to add bricks and mortar. Zebrafish is an ideal species to study the neurobiological basis of natural behaviour.
Figures and Tables
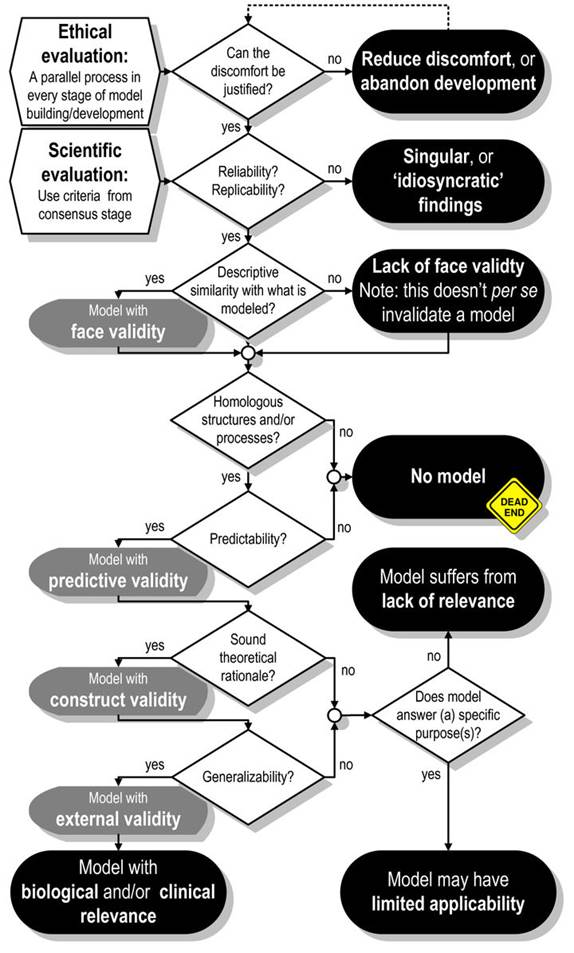
Figure 1. assessment for animal models with moral and scientific assessment standards (van der Staay, 2009)
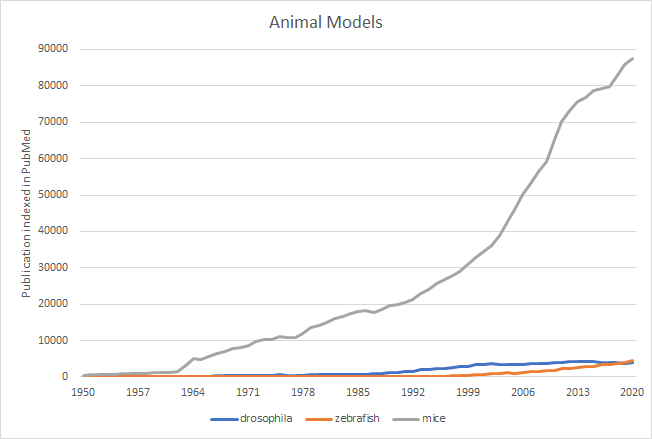
Figure 2. PubMed results by publication indexed on the date, 1950 through 2020. Each species’ search phrases contained both the scientific and popular names
Reference
Cohen BJ, Loew FM. Laboratory Animal Medicine: Historical Perspectives in Laboratory Animal Medicine 1984 Academic Press, Inc: Orlando, FL, USA; Fox JG, Cohen BJ, Loew FM (eds)
Krogh A. The Progress of Physiology. Science 1929;70:200-4.
Chatterjee, D., Gerlai, R., 2009. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res 200, 208-213
Nutton, V. (2021, January 1). Galen. Encyclopedia Britannica.
Ramirez Rozzi, F., Froment, A., 2018. Earliest Animal Cranial Surgery: from Cow to Man in the Neolithic. Scientific Reports 8.. doi:10.1038/s41598-018-23914-1
Reiter LT, Potocki L, Chien S, Gribskov M, and Bier E (2001) E A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 11:1114–1125.]
Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., Collins, J. E., Humphray, S., McLaren, K., Matthews, L., McLaren, S., Sealy, I., Caccamo, M., Churcher, C., Scott, C., Barrett, J. C., Koch, R., Rauch, G. J., White, S., Chow, W., … Stemple, D. L. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature, 496(7446), 498–503. https://doi.org/10.1038/nature12111
Kalueff, A. V., Stewart, A. M., & Gerlai, R. (2014). Zebrafish as an emerging model for studying complex brain disorders. Trends in pharmacological sciences, 35(2), 63–75. https://doi.org/10.1016/j.tips.2013.12.002
Rubinstein, A.L., 2006. Zebrafish assays for drug toxicity screening. Expert Opin Drug Metab Toxicol 2, 231-240.
Eckardt, S., McLaughlin, K. J., & Willenbring, H. (2011). Mouse chimeras as a system to investigate development, cell and tissue function, disease mechanisms and organ regeneration. Cell cycle (Georgetown, Tex.), 10(13), 2091–2099. https://doi.org/10.4161/cc.10.13.16360
Mouse Genome Sequencing Consortium, Waterston, R. H., Lindblad-Toh, K., Birney, E., Rogers, J., Abril, J. F., Agarwal, P., Agarwala, R., Ainscough, R., Alexandersson, M., An, P., Antonarakis, S. E., Attwood, J., Baertsch, R., Bailey, J., Barlow, K., Beck, S., Berry, E., Birren, B., Bloom, T., … Lander, E. S. (2002). Initial sequencing and comparative analysis of the mouse genome. Nature, 420(6915), 520–562. https://doi.org/10.1038/nature01262
McCammon, J. M., & Sive, H. (2015). Challenges in understanding psychiatric disorders and developing therapeutics: a role for zebrafish. Dis Model Mech, 8(7), 647-656. doi:10.1242/dmm.019620
Beronja, S., Janki, P., Heller, E., Lien, W.-H., Keyes, B. E., Oshimori, N., & Fuchs, E. (2013). RNAi screens in mice identify physiological regulators of oncogenic growth. Nature, 501(7466), 185-190. doi:10.1038/nature12464
Bovenkerk, B., & Kaldewaij, F. (2014). The Use of Animal Models in Behavioural Neuroscience Research. In (pp. 17-46): Springer Berlin Heidelberg.
Davidson, M. K., Lindsey, J. R., & Davis, J. K. (1987). Requirements and selection of an animal model. Isr J Med Sci, 23(6), 551-555. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3312096
Eckardt, S., McLaughlin, K. J., & Willenbring, H. (2011). Mouse chimeras as a system to investigate development, cell and tissue function, disease mechanisms and organ regeneration. Cell Cycle, 10(13), 2091-2099. doi:10.4161/cc.10.13.16360
Ericsson, A. C., Crim, M. J., & Franklin, C. L. (2013). A brief history of animal modeling. Mo Med, 110(3), 201-205. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23829102
Hajar, R. (2011). The Physician’s Little Black Bag. Heart Views, 12(1), 42-42. doi:10.4103/1995-705x.153004
Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., . . . Stemple, D. L. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature, 496(7446), 498-503. doi:10.1038/nature12111
Kalueff, A. V., Stewart, A. M., & Gerlai, R. (2014). Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci, 35(2), 63-75. doi:10.1016/j.tips.2013.12.002
Orger, M. B., & De Polavieja, G. G. (2017). Zebrafish Behavior: Opportunities and Challenges. Annual Review of Neuroscience, 40(1), 125-147. doi:10.1146/annurev-neuro-071714-033857
Pandey, U. B., & Nichols, C. D. (2011). Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery. Pharmacological Reviews, 63(2), 411-436. doi:10.1124/pr.110.003293
Romanova, E. V., & Sweedler, J. V. (2018). Animal Model Systems in Neuroscience. ACS Chemical Neuroscience, 9(8), 1869-1870. doi:10.1021/acschemneuro.8b00380
Saxena, M. (2013). Huntington’s Disease Animal Models. doi://dx.doi.org/10.13070/mm.en.3.205
Schrödinger, E. (1935). Die gegenwärtige Situation in der Quantenmechanik. Naturwissenschaften, 23(48), 807-812. doi:10.1007/BF01491891
van der Staay, F. J., Arndt, S. S., & Nordquist, R. E. (2009). Evaluation of animal models of neurobehavioral disorders. Behavioral and Brain Functions, 5(1), 11. doi:10.1186/1744-9081-5-11
Waterston, R. H., Lindblad-Toh, K., Birney, E., Rogers, J., Abril, J. F., Agarwal, P., . . . Lander, E. S. (2002). Initial sequencing and comparative analysis of the mouse genome. Nature, 420(6915), 520-562. doi:10.1038/nature01262
Wessler. (1976). Introduction : what is a model? . In Animal models of thrombosis and hemorrhagic diseases.
Animal Model Systems in Neuroscience Elena V. Romanova and Jonathan V. Sweedler ACS Chemical Neuroscience 2018 9 (8), 1869-1870 DOI: 10.1021/acschemneuro.8b00380
Wright, C. (2002). Animal models of depression in neuropsychopharmacology qua Feyerabendian philosophy of science. In S. P. Shohov (Ed.), Advances in psychology research, Vol. 13, pp. 129–148). Nova Science Publishers.
Morgan, Thomas H. “Random Segregation Versus Coupling in Mendelian Inheritance.” Science (1911): 384. http://science.sciencemag.org/content/34/873/384
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. (2000) The genome sequence of Drosophila melanogaster. Science 287:2185–2195.
Reiter LT, Potocki L, Chien S, Gribskov M, and Bier E (2001) E A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 11:1114–1125.


